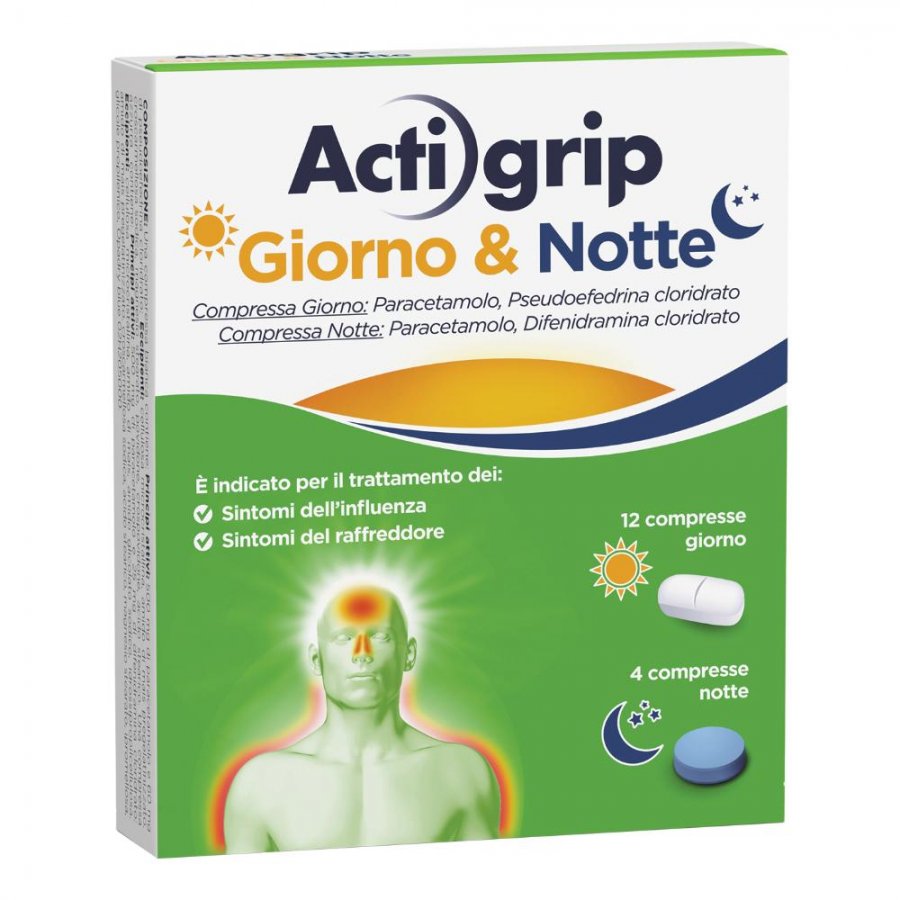
Actigrip Giorno e Notte is a non-prescription medicine in tablets with a double formulation based on paracetamol, pseudoephedrine and diphenhydramine. Actigrip Giorno e Notte fights painful and flu symptoms during the day to stay active and decongests the lower respiratory tract to promote rest during the night hours in case of colds and stuffy nose.
The 12 white tablets of Actigrip Giorno e Notte are taken during the day and have a specific anti-fever, analgesic and decongestant action. They help you stay active during the day by fighting the symptoms of fever and cold.
The 4 blue tablets of Actigrip Giorno e Notte are taken at night. They continue the antipyretic and analgesic action of paracetamol and add the antihistamine action of diphenhydramine which helps to clear the nose and lower airways to breathe better at night and rest well.
One white tablet of Actigrip Day and Night contains:
- Paracetamol (500 mg) : Antipyretic, decongestant. Helps manage painful symptoms and reduces fever.
- Pseudoephedrine hydrochloride (60 mg) : Acts as a nasal decongestant , relieves nasal congestion and reduces swelling of the nasal membranes making breathing easier.
One Actigrip Day and Night midnight blue tablet contains:
- Paracetamol (500 mg) : Antipyretic, decongestant. Helps to manage painful symptoms and reduces fever.
- Diphenhydramine hydrochloride (25mg) : Helps reduce cold symptoms such as sneezing and runny nose. Diphenhydramine is an antihistamine that helps you get a good night's sleep by relieving flu symptoms and clearing the lower airways.
ACTIVE INGREDIENTS
Active ingredients contained in Actigrip Giorno e Notte Tablets 12+4 - What is the active ingredient in Actigrip Giorno e Notte Tablets 12+4?
One white tablet (day) contains; active ingredients: paracetamol 500 mg, pseudoephedrine hydrochloride 60 mg. One blue coated tablet (night) contains; active ingredients: paracetamol 500 mg, diphenhydramine hydrochloride 25 mg. For the full list of excipients, see section 6.1.
EXCIPIENTS
Composition of Actigrip Day and Night Tablets 12+4 - What does Actigrip Day and Night Tablets 12+4 contain?
One white tablet (day) contains: microcrystalline cellulose, pregelatinised maize starch, croscarmellose sodium, magnesium stearate, povidone, crospovidone, stearic acid. One blue coated tablet (night) contains: microcrystalline cellulose, maize starch, sodium starch glycolate, hydroxypropylcellulose, pregelatinised maize starch, croscarmellose sodium, stearic acid, magnesium stearate, hypromellose, Opadry blue 02H205000 (containing propylene glycol).
DIRECTIONS
Therapeutic indications Actigrip Day and Night Tablets 12+4 - Why is Actigrip Day and Night Tablets 12+4 used? What is it for?
Treating cold and flu symptoms.
CONTRAINDICATIONS
Contraindications Actigrip Day and Night Tablets 12+4 - When should Actigrip Day and Night Tablets 12+4 not be used?
Therapeutic indications Actigrip Day and Night Tablets 12+4 - Why is Actigrip Day and Night Tablets 12+4 used? What is it for?
/SECONDARY EFFECTS Hypersensitivity to the active substances or to any of the excipients listed in paragraph 6.1; children under 12 years; confirmed or suspected pregnancy, breastfeeding; cardiovascular disease, hypertension; patients with a history of stroke or predisposing risk factors; diabetes; glaucoma; gastrointestinal tract stenosis; hyperthyroidism; prostatic hypertrophy, urogenital tract stenosis; asthma; in patients undergoing treatment with monoamine oxidase inhibitors (MAOIs) and in the two weeks following such treatment; patients with a history of convulsions, epilepsy. Furthermore, due to the paracetamol content, ACTIGRIP DAY & NIGHT is contraindicated in patients with manifest glucose-6-phosphate dehydrogenase insufficiency.
DOSAGE
Quantity and method of taking Actigrip Giorno e Notte Tablets 12+4 - How to take Actigrip Giorno e Notte Tablets 12+4?
Dosage. Adults and children over 12 years of age: one white tablet three times a day (in the morning, at noon and in the afternoon) plus one blue tablet in the evening before bedtime. After 3 days of continuous use without appreciable results or if high fever or other side effects appear, discontinue treatment. Do not exceed the recommended doses. Method of administration: take the tablets orally, without chewing them.
CONSERVATION
Storage Actigrip Day and Night Tablets 12+4 - How to store Actigrip Day and Night Tablets 12+4?
Store below 25 degrees C. Store in the original package to protect from light and moisture.
WARNINGS
Warnings Actigrip Day and Night Tablets 12+4 - About Actigrip Day and Night Tablets 12+4 it is important to know that:
The use of ACTIGRIP DAY & NIGHT should be avoided in conjunction with analgesics and antipyretics. High doses or prolonged administration of paracetamol, present in the medicinal product or in other drugs containing paracetamol, can cause high-risk liver disease, even serious alterations to the kidney and blood and other serious adverse reactions. In adults and children over 12 years of age, the total dose of paracetamol should not exceed 4 g per day. Paracetamol should be administered with caution to patients with mild to moderate hepatocellular insufficiency (including Gilbert's syndrome), severe hepatic insufficiency (Child-Pugh > 9), acute hepatitis, in concomitant treatment with drugs that alter liver function, glucose-6-phosphate dehydrogenase deficiency, haemolytic anaemia. In case of concomitant treatment with anticoagulant or antiplatelet drugs, the doses of ACTIGRIP DAY & NIGHT must be reduced. Skin safety: Serious skin reactions such as acute generalized exanthematous pustulosis (AGEP), Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have been reported very rarely in patients treated with paracetamol. Patients appear to be at higher risk in the early stages of therapy: the onset of the reaction occurs in most cases early in treatment. Serious skin reactions such as acute generalized exanthematous pustulosis (AGEP) may occur with products containing pseudoephedrine. This acute pustular eruption may occur within the first 2 days of treatment, with fever and numerous, small, mostly non-follicular pustules arising from a widespread oedematous erythema and located mainly on the skin folds, trunk and upper limbs. Patients should be carefully monitored. If signs and symptoms such as pyrexia, erythema or numerous small pustules are observed, the administration of ACTIGRIP DAY & NIGHT should be discontinued and appropriate measures should be taken if necessary (see section 4.8). Patients should be informed of the signs of serious skin reactions. ACTIGRIP DAY & NIGHT should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity. Do not use with any other product containing diphenhydramine, including those for topical use. Cardiovascular and cerebrovascular safety. Patients should be informed that treatment should be discontinued if the following symptoms associated with the pseudoephedrine content occur: arterial hypertension, tachycardia, palpitations or cardiac arrhythmia, nausea or any neurological signs (onset or exacerbation of headache). Ischemic colitis: some cases of ischemic colitis have been reported with medicinal products containing pseudoephedrine. If sudden abdominal pain, rectal tenesmus, rectal bleeding or other symptoms of ischaemic colitis develop (see section 4.8), pseudoephedrine should be discontinued and a doctor should be consulted. Ischaemic optic neuropathy: Cases of ischaemic optic neuropathy have been reported with pseudoephedrine. Pseudoephedrine should be discontinued if sudden loss of vision or reduction in visual acuity occurs, for example in the case of scotoma. If symptoms persist or worsen or if new symptoms appear, patients should stop using the medicinal product and consult a doctor. In rare cases of allergic reactions, ACTIGRIP DAY & NIGHT should be discontinued. Paracetamol should be used with caution in subjects with renal insufficiency or liver problems. Subjects suffering from alcoholism should consult their doctor before taking paracetamol or other analgesics or antipyretics. Patients with the following respiratory conditions should be advised to consult a doctor before using defenhydramine: emphysema, chronic bronchitis. They should not take pseudoephedrine unless the doctor considers it necessary: patients with reduced renal function and patients with thyroid disease; do not use in patients suffering from hyperthyroidism (see section 4.3). Due to the paracetamol content, the administration of ACTIGRIP DAY & NIGHT may interfere with the determination of uric acid (using the phosphotungstic acid method) and with that of glycaemia (using the glucose-oxidase-peroxidase method). For those who play sports: the use of the drug without therapeutic need constitutes doping and may in any case cause positive anti-doping tests. ACTIGRIP DAY & NIGHT may cause drowsiness. See section 4.8 Undesirable effects.
INTERACTIONS
Interactions Actigrip Giorno e Notte Tablets 12+4 - Which medicines or foods can modify the effect of Actigrip Giorno e Notte Tablets 12+4?'
Concomitant use of ACTIGRIP DAY & NIGHT with tricyclic antidepressants, sympathomimetics (e.g. decongestants, appetite suppressants and amphetamine-like agents), or with MAOIs may interfere with the catabolism of catecholamines and may occasionally cause increases in blood pressure. Acute hypertensive crises with concomitant use of MAOIs and sympathomimetic amines have been reported in medical literature. The effects of anticholinergic drugs (e.g. atropine and other psychotropic drugs) may be potentiated by concomitant use of ACTIGRIP DAY & NIGHT. Patients should be informed that additive effects may occur with alcohol, hypnotics, sedatives and tranquilizers and therefore they should not be taken concomitantly. Use with extreme caution and under close supervision during chronic treatment with drugs that can determine the induction of hepatic monooxygenases or in case of exposure to substances that can have this effect (for example rifampicin, cimetidine, antiepileptics such as glutethimide, phenobarbital, carbamazepine). The use of antihistamines at the same time as certain ototoxic antibiotics can mask the first signs of ototoxicity, which may reveal itself only when the damage is irreversible. The habitual intake of anticonvulsant drugs or oral contraceptives can, by an enzyme induction mechanism, accelerate the metabolism of paracetamol, decreasing the plasma concentration, increasing the rate of elimination. The rate of absorption of paracetamol can be increased by the concomitant intake of metoclopramide and domperidone and decreased by cholestyramine. Treatment with probenecid may lead to a decrease in the clearance of paracetamol and an increase in its half-life in the blood. Use of the product is not recommended if the patient is being treated with antipyretics and other analgesics (NSAIDs, selective COX-2 inhibitors, corticosteroids). Due to the pseudoephedrine content, the effect of antihypertensives that interfere with sympathetic activity (e.g. methyldopa, alpha and beta blockers, debrisoquine, guanethidine, bethanidine and bretylium) may be partially cancelled out by ACTIGRIP GIORNO & NOTTE, which should therefore not be taken at the same time. Anticoagulants such as warfarin and other coumarins should not be taken at the same time as ACTIGRIP GIORNO & NOTTE as their effect may be increased by prolonged use of paracetamol with a greater risk of bleeding. Cases of high anion gap pyroglutamic acid metabolic acidosis (5-oxoprolinemia) have been reported with concomitant use of therapeutic doses of paracetamol and flucloxacillin. Patients reported to be most at risk are elderly women with underlying conditions such as sepsis, abnormal renal function and malnutrition. Most patients improve after discontinuation of one or both drugs. Before taking ACTIGRIP DAY & NIGHT, patients should ask their doctor if they are taking the antibiotic flucloxacillin.
SIDE EFFECTS
Like all medicines, Actigrip Giorno e Notte Tablets 12+4 can cause side effects - What are the side effects of Actigrip Giorno e Notte Tablets 12+4?
The following are the side effects recorded following the intake of ACTIGRIP DAY & NIGHT. The frequency of side effects is defined using the following convention: very common (>=1/10); common (>=1/100 and <1/10); uncommon (>=1/1,000 and <1/100); rare (>=1/10,000 and <1/1,000); very rare (<1/10,000); not known (cannot be estimated from the available data). Very common side effects. Gastrointestinal disorders: abdominal or stomach pain, dyspepsia, diarrhoea and vomiting, dry throat. Nervous system disorders: headache, drowsiness, sedation, excitation, increased sweating, sleep disturbances. Eye disorders: altered vision. Skin and subcutaneous tissue disorders: skin rashes, urticaria. Respiratory, thoracic and mediastinal disorders: dry nose. Common side effects. Skin and subcutaneous tissue disorders: pruritus, contact dermatitis, inflammation of the skin or mucous membranes. Cardiac disorders: orthostatic/postural hypotension, arrhythmia, tachycardia. Nervous system disorders: tinnitus, ataxia, euphoria and tremors, hypotension, decreased mucous secretions. Eye disorders: diplopia, impaired vision, glaucoma, angle-closure glaucoma. Gastrointestinal disorders: epigastric disorders. Respiratory, thoracic and mediastinal disorders: dyspnoea. Renal and urinary disorders: urinary retention. Metabolism and nutrition disorders: hyperamylasemia. Systemic disorders and administration site conditions: fatigue, asthenia. Hepatobiliary disorders: liver function disorders. Uncommon side effects. Skin and subcutaneous tissue disorders: fixed drug eruption (FDE), erythema multiforme, exanthemas. Renal and urinary disorders: acute renal failure, interstitial nephritis, haematuria, anuria. Gastrointestinal disorders: constipation. Respiratory, thoracic and mediastinal disorders: sneezing, dryness of the pharynx and bronchial tree. Skin and subcutaneous tissue disorders: photosensitivity. Nervous system disorders: central depression, mental confusion, cognitive function disorders. Rare side effects. Endocrine disorders: hyperthyroidism. Renal and urinary disorders: renal papillary necrosis. Nervous system disorders: hallucinations and nightmares, secondary mania, anxiety, psychiatric disorders, severe headache, memory or concentration impairment, convulsions. Blood and lymphatic system disorders: blood dyscrasias, agranulocytosis, anaemia, haemolytic anaemia, thrombocytopenia. Immune system disorders: anaphylactic shock, laryngeal edema. Hepatobiliary disorders: hepatitis. Gastrointestinal disorders: pancreatitis. Very rare adverse reactions. Blood and lymphatic system disorders: leukopenia, neutropenia, pancytopenia. Cardiac disorders: angina, ST segment elevation, myocardial infarction, hypertension, oedema. Skin and subcutaneous tissue disorders: angioedema. Hepatobiliary disorders: hepatotoxicity. Immune system disorders: toxic shock syndrome. Unknown adverse reactions. Nervous system disorders: confusional state, irritability, agitation, abnormal coordination, paraesthesia, psychomotor hyperactivity, feeling nervous, cerebrovascular accident*. Investigations: increased transaminases, increased blood pressure. Skin and subcutaneous tissue disorders: pruritic rash, severe skin reactions, including acute generalised exanthematous pustulosis (AGEP) (see section 4.4). Renal and urinary disorders: dysuria. Respiratory, thoracic and mediastinal disorders: chest discomfort. Cardiac disorders: palpitations. Immune system disorders: hypersensitivity. Psychiatric disorders: visual hallucination. Gastrointestinal disorders: ischemic colitis* (see section 4.4). Eye disorders: ischemic optic neuropathy. *adverse reactions collected during post-marketing experience with pseudoephedrine. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system at http://www.agenziafarmaco.gov.it/content/comesegnalare-una-sospetta-reazione-avversa.
PREGNANCY AND BREASTFEEDING
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking Actigrip Giorno e Notte Tablets 12+4. The medicine is contraindicated in cases of confirmed or suspected pregnancy, and during breast-feeding. There are no adequate and controlled clinical studies in pregnant or breast-feeding women for the combination of diphenhydramine, paracetamol and pseudoephedrine. Pregnancy: diphenhydramine crosses the placenta and is excreted in breast milk, but the levels have not been reported. Paracetamol crosses the placenta. Breast-feeding: paracetamol, pseudoephedrine and diphenhydramine are secreted in breast milk; therefore, ACTIGRIP GIORNO & NOTTE is contraindicated during breast-feeding. Paracetamol is excreted in breast milk in low concentrations (from 0.1% to 1.85% of the dose ingested by the mother). Pseudoephedrine is distributed in breast milk; within 24 hours, up to 0.6% of a single 60 mg dose can be detected in breast milk. No data on plasma protein binding are available in humans. Data from a study of 8 nursing mothers taking 60 mg pseudoephedrine every 6 hours suggest that approximately 4.3% of the maximum daily dose (240 mg) could be made available to the infant by the nursing mother."

























